Ticagrelor: the World’s First Reversible Combination Orally-administered P2Y12 Adenosine Diphosphate Receptor Antagonist
![]()
Ticagrelor, a new platelet aggregation inhibitor, is the world’s first reversible combination orally-administered P2Y12 adenosine diphosphate receptor antagonist. It is used to reduce cardiovascular death and heart attacks in patients with acute coronary syndrome. Ticagrelor is white to off-white powder with hygroscopic, so low temperature storage and transport is required.
Mechanism
Ticagrelor has reversibly acts on the 2 purine receptor subtype P2Y12, of vascular smooth muscle cells (VSMC,) which does not require metabolic activation, and has a significant inhibitory effect on platelet aggregation induced by adenosine diphosphate. In this case, platelet function will recover rapidly after stopping the dose.
Features
- Rapid onset of action
Ticagrelor can be absorbed in approximately 1.5 hours and After about 2.5 hours, ticagrelor can rapidly transformed into circulating metabolite AR-C124910XX. The mean bioavailability of ticagrelor was approximately 36%.
- Powerful drug
Without increasing major bleeding, ticagrelor treatment for 12 months significantly reduced the risk of cardiovascular death by 21% compared with clopidogrel. Ticagrelor is already a first recommended drug for cardiovascular disease in Europe.
- Platelet function recovered quickly
The interaction between ticagrelor and platelet P2Y12 ADP receptor is reversible, without conformational change and signal transmission, and platelet function in blood recovers rapidly after drug withdrawal.
Sythesis
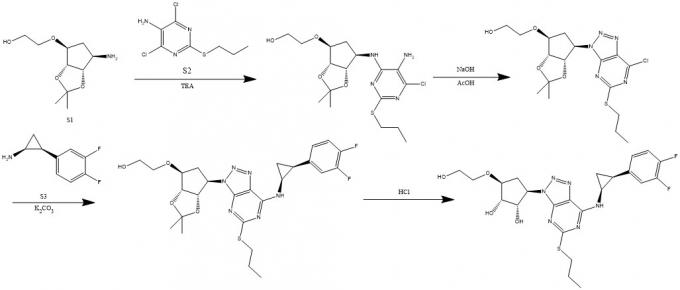
Ticagrelor is mainly composed of three major fragments: a five-membered ring, a thioether pyrimidine, and a three-membered ring. At present, a variety of synthesis routes have been reported, and the common point is that there is no construction of chiral centers in the synthesis process, and all the six chiral centers are introduced by starting materials.
In this synthetic route, starting materials S1 and S2 were coupled, then the ring was diazosed off under the conditions of sodium nitrite and acetic acid, and then coupled with starting material S3. Finally, the finished product of ticagrelor was obtained by removing the forkone protection.
Usage and dosage
Oral administration. This product can be taken before or after meals.
The initial dose was a single dose of 180 mg, followed by 90 mg twice a day.
It should be used in combination with aspirin unless clearly contraindicated. After the initial loading dose of aspirin, aspirin was maintained at a dose of 75 to 100mg once daily.
Ticagrelor can be started in ACS patients who have already received a loading dose of clopidogrel.
Treatment may be given for up to 12 months unless there is a clinical indication to discontinue treatment.
Cautions
The most frequently reported adverse effects were dyspnea, contusion, and epistaxis in patients who received ticagrelor, which occurred more frequently than in patients who received clopidogrel. Among patients with acute coronary syndromes who are treated with ticagrelor combined with aspirin, there is an increased risk of bleeding. Therefore, the increase of bleeding risk should be balanced against the benefit of preventing atherothrombotic events. Interruptions of ticagrelor tablets should be avoided. If ticagrelor must be temporarily discontinued, treatment should be restarted as soon. The risks of myocardial infarction, thrombosis, even death would increase if ticagrelor is discontinued.



